Chemokines
The chemokine family
To protect from pathogens and tissue damage, leukocytes must migrate to sites of infection or injury. This process is regulated, in the main, by members of the chemokine (chemotactic cytokine) family of peptides (1). Typically, chemokines are secreted by injured or infected tissue. Leukocytes circulate in the blood and upon chemokine binding, roll along the endothelium and migrate into the tissue.
Chemokines are a vertebrate 'invention' and the most ancient chemokine is CXCL12 (2). It is likely that the original role for CXCL12 was to regulate stem cell migration during early vertebrate embryogenesis (3, 4). From this single chemokine, the chemokine family has now evolved to the point where humans have at least 45 different chemokines which are involved in regulating leukocyte migration (2).
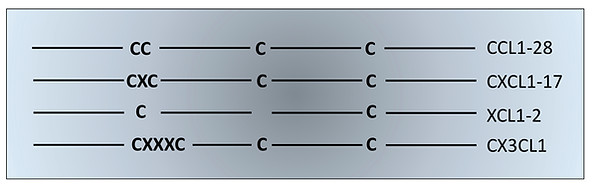
Chemokines are defined by the presence of conserved cysteine residues and are grouped based on the position of these cysteines. These are CC (28 members), CXC (17 members), XC (2 members) and CX3C (1 member)
Chemokines have homeostatic and inflammatory roles
Chemokine receptors
Chemokines mediate their effects via G protein coupled receptors (7). These are classed as CCRs (10 members) CXCRs (6 members), XCR (1 member) and CX3CR (1 member), based on the group of chemokines they bind. Like chemokines, these receptors can be classified as being inflammatory or homeostatic according to the contexts in which they function and the chemokines they bind. The homeostatic receptors typically bind one, or at most two, chemokines and these chemokines only bind to one receptor. It is presumed that this ensures targeted and precise navigation of cells bearing these receptors.
The inflammatory receptors can bind to several different chemokines, for example CCR3 can bind at least 9 CC chemokines and CCR5 binds CCL3, 4, 5 and 8. In turn, each of these ligands can bind to multiple receptors with CCL3, for example, binding to CCR1 and CCR5 (8). It is presumed that this apparent redundancy of receptor and ligand function in inflammation is important for ensuring a robust innate immune response to tissue insult. However, this complexity has made understanding the orchestration of inflammatory responses very difficult! Part of our work focuses on understanding the function of CCR1, CCR2, CCR3 and CCR5 at rest and during inflammation.
Atypical chemokine receptors
References
1. Rot, A., and von Andrian, U.H. (2004) Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol 22:891-928.
2. Zlotnik, A., Yoshie, O., and Nomiyama,H. (2006) The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol 7:243.
3. Doitsidou, M., Reichman-Fried, M.,Stebler, J., Koprunner, M., Dorries, J., Meyer, D., Esguerra, C.V., Leung, T.,and Raz, E. (2002)Guidance of primordial germ cell migration by the chemokine SDF-1. Cell 111:647-659.
4. Boidajipour, B., Mahabaleshwar, H.,Kardash, E., Reichman-Fried, M., Blaser, H., Minina, S., Wilson, D., Xu, Q.L.,and Raz, E. (2008) Control of chemokine-guided cell migration by ligand sequestration. Cell 132:463-473.
5. Mantovani, A. (1999) The chemokine system: redundancy for robust outputs. Immunol Today 20:254-257.
6. Zlotnik, A., and Yoshie, O. (2000) Chemokines: a new classification system and their role in immunity. Immunity 12:121-127.
7. Murphy, P.M., Baggiolini, M., Charo,I.F., Hebert, C.A., Horuk, R., Matsushima, K., Miller, L.H., Oppenheim, J.J.,and Power, C.A. (2000) International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev 52:145-176
8. Zlotnik, A and Yoshie, O. (2012). The chemokine superfamily revisited. Immunity. 36:705-716.
9. Rot, A. (2005) Contribution of Duffy antigen to chemokine function. Cytokine Growth Factor Rev 16:687-694.
10. Graham, G.J., and Locati, M. (2013) Regulation of the immune and inflammatory responses by the 'atypical' chemokine receptor D6. Journal of Pathology 229:168-175.
11. Bonecchi, R. and Graham, G. J. (2016). Atypical chemokine receptors and their roles in the resolution of the inflammatory response. Front. Immunol. 7, 224.



Chemokines can act as regulators of either inflammatory or homeostatic leukocyte migration (5,6). Inflammatory chemokines control the movement of leukocytes to sites of damage or infection. These are not normally expressed at high levels but are induced rapidly following tissue insult or injury. In contrast, the homeostatic chemokines are mainly involved indirecting the basal trafficking of leukocytes to peripheral tissues and secondary lymphoid organs, such as the spleen and lymph nodes.


In addition to the classical signalling chemokine receptors, there is a smaller family of atypical chemokine receptors that do not signal through G proteins. This family includes ACKR1 (9), ACKR2 (10) ACKR3 and ACKR4 (11). An interesting review of atypical chemokine receptors and their functions can be found here. Another part of our work aims to understand the role of ACKR2 in embryonic and adult development as well as in diseases such as cancer.
